Hexavalent chromium in cement, 15 years later
The object of this paper is to review, more than fifteen years after the introduction of directive 2003/53/EC, the technological aspects of the formation and reduction of soluble chromates in Portland cements.
Since January 2005, following the implementation of directive 2003/53/EC [1], cement or cement-based preparations placed on the European market must not release, when mixed with water, more than 2 mg/kg (ppm – part per million) of soluble hexavalent chromium. Control of Cr(VI) is usually achieved by the addition of suitable chemical additives to the cement. These chemicals are able to reduce chromium to the trivalent state thanks to an electrochemical red-ox reaction that takes place when cement is mixed with water.
European cement producers and in general every company (even if located outside the EU) that intends to sell cement or cement-based materials in Europe must comply with this regulation. Moreover, the storage conditions and the storage period appropriate to maintaining the activity of the reducing agent and to keeping the content of soluble hexavalent chromium below the limit must be declared on the packaging and in the technical documentation [1]. This particularly affects the cement imported into Europe, where the delivery time may delay the moment of cement use.
Background: reasons behind the 2003/53/EC
The European Directive on hexavalent chromium in cements was released with the purpose of reducing the incidence of allergic contact dermatitis, a professional disease in masonry workers that was quite common before 2005.
Allergic contact dermatitis (also known as cement eczema) was first reported in 1908, when a significant percentage of the workers involved in the Paris Metro system experienced eruption of the hands [2]. In addition to the highly alkaline conditions of cement mixing water, hexavalent chromium was recognized to be the main reason for the incidence of allergic dermatitis when specific “patch tests” were performed. This was correlated to the presence of hexavalent chromium in Portland clinkers and cements [3, 4].
Without any measure to reduce the content of chromates or to avoid contact with cement, the incidence of dermatitis in masonry workers could reach high levels [5], representing a serious professional disease. This is the reason why the European Union, following the experience of some specific countries (such as Denmark and Finland), concluded that a limitation on the level of hexavalent chromium in cements could be useful and envisaged the addition to cements of suitable reducing agents.
Recent studies demonstrated that the incidence of allergic contact dermatitis related to Cr(VI) has been decreasing since control of chromates in cements started. Moreover, in several countries outside Europe, there is an increasing consciousness about the need to introduce similar limitations. For example, specific epidemiological investigations have recently been published for Israel [6] and Australia [7]. It can be concluded that the impact of the European Directive on hexavalent chromium has been positive.
Formation of hexavalent chromium in cement
Basic facts of chromium chemistry allow us to understand the reason why modern Portland cements contain soluble chromates and what could be the strategy to control their formation. Chromium (Cr) is a transition metal and may exist in different oxidation states, mainly zero, +3 and +6. Immediately next to chromium, in the same period of the Mendeleev’s periodic table of elements, manganese and iron make an appearance. In fact, these elements (Cr, Mn, Fe) are widely used together in many alloys, suggesting some similarities. Moreover, in nature Cr is mainly found as mixed iron-chromium oxide (in the mineral chromite), as a confirmation of the chemical affinity with iron.
The diagram pH/Red-Ox potential (Pourbaix diagram [8], Figure 1) of chromium describes in detail the stability area of different compounds. Although this diagram relates to equilibria in aqueous solution, the information contained can to some extent be used to describe chromium behavior in different conditions. It can be clearly seen that the Red-Ox potential of the couple Cr6+/Cr3+, although high in acid media (hence Cr6+ is a strongly oxidizing agent), it decreases with pH and in alkaline conditions the trivalent chromium is easily oxidized to the hexavalent form. The stability area of chromate broadens at high pH. The last point to be considered: a similarity exists between chromate and sulphate ion, in terms of electrical charge and coordination structure of oxygen atoms.
There are enough details to describe what happens during cement manufacturing:
Raw materials for clinker production (typically iron ore, but also bauxite, iron scraps, waste from metallurgical industries and, last but not least, fuels, both traditional and alternative) contain chromium as a metal or in trivalent state.
Total chromium gets into contact with oxygen in the highly alkaline media found inside the kiln. The combination of free lime, excess of oxygen and high temperature promotes the partial oxidation of chromium to the hexavalent form. The kinetics of the process are not easy to describe as they involve the diffusion of oxygen in the semi-molten mass flowing through the kiln.
Thanks to similarities in charge and structure, the chromate probably behaves similarly to sulphate, hence it reacts with alkalis to give highly soluble potassium or sodium chromate.
Oxidation of Cr(III) and fixation as soluble chromate can be described by the following reaction:
4Cr2O3 + 6O2 + 8K2O = 8K2CrO4
The simple model described above is coherent with many known facts, listed here below:
The higher the iron content of clinker, the higher is the soluble chromium. White clinkers (with little or no iron) do not contain soluble chromates.
The use of iron scrap or metal-related waste materials (foundry sand, steel converter slag, incinerator slag, by-products of steel industry) usually promotes higher levels of hexavalent chromium.
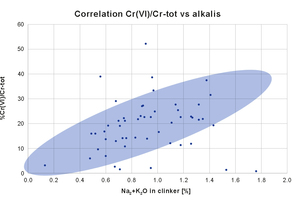
There is a direct correlation between alkalis and soluble chromates.
Figure 2 reports a series of Mapei internal data, showing the ratio hexavalent to total chromium versus alkalis content, for several cements. Generally speaking, there is a direct correlation between alkalis and the formation of soluble chromates (blue area), but indeed there are cases where the level of Cr(VI) is lower than expected (and the reason may be linked to milder oxidizing conditions in the kiln), and cases where the formation of chromates is higher than expected (and it can supposed that oxidizing conditions are harsher than usual).
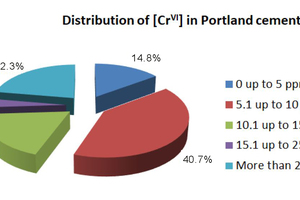
A distribution of Cr(VI) levels in commercial clinkers and cements (not treated with reducing agents) is described in Figure 3 (Mapei internal data). Many cements have Cr(VI) ranging from 0 to 15 ppm, but it is not uncommon to have situations in which the Cr(VI) level is extremely high, above 25 ppm and reaching 90-100 ppm in some cases.
Considering what has been described above, the level of hexavalent chromium in clinker and cement could be controlled and strongly reduced through an accurate selection of raw materials. Obviously, this has a cost (since the use of waste materials is driven by economic considerations) that should be balanced with the cost for the use of chemical reducing additives.
Technologies for the reduction of soluble chromates
Going back to the Pourbaix diagram of chromium (Figure 1), it can be noticed that reduction of the hexavalent form to trivalent is easy in acid media but becomes more difficult as the pH increases. In the cement mixing water (where the pH is fixed between 12.5 and 13.5 depending on the calcium hydroxide buffer effect and level of alkalis), the Red-Ox potential of the couple Cr6+/Cr3+ is close to zero, hence intermediate between oxidizing and reducing agents. Hence, from thermodynamics it is known that complete and efficient reduction of soluble chromates in alkaline media can be obtained only through the use of strong reducing agents having very negative Red-Ox potential. Moreover, there are several kinetics considerations that further limit the chemicals that can be used for elimination of chromates in cements. A detailed description of the theory behind this has been provided in other publications [10].
The content of chromates in cements can be reduced using three main technologies currently available:
1. Iron(II) compounds (mainly ferrous sulphate)
2. Tin(II) compounds (mainly stannous sulphate)
3. Antimony(III) compounds
A comparison of these three technologies in terms of dosages and use is summarized in Table 1.
Iron(II) compounds
Ferrous sulphate is probably the most common reducing agent used in cements, mainly thanks to low cost and high availability (although periodical shortage situations have been reported and availability in the near future will probably be further reduced due to higher consumption). On the other hand, the stability in conditions of high temperature and high humidity (as happens in grinding mills or during storage) is poor. Part of the Fe(II) is oxidized during grinding and the dosage needs to be increased if a long term reduction is to be assured. Hence, efficacy dosage is strictly related to characteristics of the grinding plant and may vary from 90 to more than 500 g/t per ppm of Cr(VI). Higher dosages are required in the case of high mill temperature, high moisture and high ratio of reject to fresh feed (in the case of high circulating load, the longer residence time in the harsh condition of the mill promotes the oxidation and deactivation of Fe2+). In the case of cements with extremely high chromates content (as happens quite often when waste materials are used as correctors for clinker manufacturing), iron sulphate may be unable to completely reduce chromium for the required time. Side effects such as red/brown spots (due to oxidized Fe3+) have been described in some cases. These can be reduced using a suitable dosage control and an appropriate granulometry of the ferrous sulphate.
Tin(II) compounds
Stannous sulphate can be used as powder, more or less free flowing if formulated with anti-caking and moisture absorber agents, or in the form of liquid additive. Efficacy, dosage, and stability of the reduction are very good, but the cost is prohibitive for normal applications and, for special binders such as calcium sulpho-aluminate or calcium aluminate cements, the efficacy of tin as a reducing agent can be lower.
Antimony(III) compounds
Antimony(III) compounds as reducing agents for chromates in cement have been widely described in several publications and patents [11, 12] and, when launched onto the market, represented a breakthrough technology due to very high efficacy and lower cost than tin sulphate.
Dosages can be carefully controlled due to the extreme stability of antimony and the reducing activity remains unchanged for several months. Thanks to the combination of technical performances and cost (although subjected to fluctuations, there is a remarkable saving in comparison to tin), antimony compounds are the ideal solution when long reduction efficacy must be guaranteed.
Reducing agents based on antimony are available in the market as liquid additive (for addition during cement grinding) and in the form of powder additive for addition during grinding or packaging of cement. Powder products can also be obtained as micro-granules with antimony encapsulated in a suitable soluble polymer, in a sort of controlled release technology that reduces the level of fine dusts that are potentially harmful when inhaled.
Safety in the use of reducing agents
When discussing the advantages and disadvant-ages of any technology, the aspects related to safety in practical use is one of the most important. The safety profile (according to the European Regulation CLP 1272/2008 on the Classification, Labeling and Packaging of chemical substances) is summarized in Table 2 [13].
Antimony trioxide is suspected of causing cancer, and this risk is related to the inhalation of fine particles [14]: the parameter normally used in industrial hygiene to assess the risk when inhaled is the TLV – Threshold Limit Value (it is basically the highest allowed concentration of a specific chemical in the atmosphere of the working environment, either expressed as average over a period of time, or as a maximum level [15]). Several determinations of antimony concentrations in the atmosphere of a cement plant during normal operations that involve the use of commercially available antimony-based reducing agents have been performed. For example, the levels of antimony (expressed as mg/m3) close to the mill during cement grinding and during packaging and use (concrete or mortar preparation) of cement produced with antimony-based reducing agents are normally below the detection limit, while the TLV-TWA (Time Weighted Average) of antimony is 0.5 mg/m3. This is obvious if one considers that Portland cement has its own TLV-TWA limit, in the range of 1 mg/m3 for the respirable fraction. Considering that dosages of antimony with respect of cement are around hundreds of ppm, if the cement TLV is respected automatically the antimony is far below the maximum allowed limit.
Having said this, it is anyway recommended to avoid or reduce as far as possible any risk of fine dust release, to eliminate the theoretical risk of dust inhalation. This is the reason why commercial products based on antimony trioxide are usually in the form of liquid dispersion. When a powder product is needed, suitable encapsulation technologies that reduce the fine fractions are available, as summarized in Table 3.
Conclusions
The introduction of the European Directive that limits the level of soluble hexavalent chromium in cement and cement-based materials has represented, during the last fifteen years, a significant challenge that the cement industry has been facing. Nevertheless, it ended up in a global improvement of health and safety in the building sector. It is now regarded as a model for other countries where similar limitations are not present. The theory behind the formation and elimination of oxidized chromium (reviewed in the present paper) is well understood and the technologies available for the reduction of Cr(VI) are now mature. The commercial supply of reducing additives can sustain all the requirements of the cement industry.


![1 Red-Ox potential/pH diagram of chromium (Pourbaix diagram [8]); reproduced from [9]](/uploads/images/2022/w300_h200_x600_y554_Materials_Magistri_Fig_1_Pourbaix-b77f7dea98cacd1b.jpeg)