The quicker, the better: how automated isothermal calorimetry (polabCal) improves quality and process control in cement plants
Future green cements will be produced from less clinker; and the clinker will be produced with new technologies releasing less and at someday zero CO2. Cement suppliers will be challenged to maintain consistent clinker reactivity at a lower CaCO3 content in the raw materials and with fuels emitting less CO2. Finally, at the construction site the performance of future green cements should be compatible with current working practice and time schedules. In future composition and fineness data might not be sufficient to maintain product consistency of clinker and cement. New automated isothermal calorimetry analyzers provide on a routine basis data on early hydration reactions during the first 60 minutes of hydration. Fast reactivity data facilitates a rapid feed-back to the process to correct set-point deviations. Automated calorimetry rapidly gives clear indications on how to improve product consistency without waiting for delayed and error prone compressive strength testing. Clinker and cement production with a focus on reactivity will lead to new levels of product consistency at minimum safety margins. This paper provides an overview from conventional isothermal calorimetry to automated isothermal calorimetry, and it outlines the capabilities of this new tool with practical examples for composite cement production, clinker production, sulfatization of cement and cement additive application. The accuracy and repeatability of this new method opens doors for new process control cycles in cement plants. The control of reactivity of both clinker and cement is the key for optimized kiln operation for the production of low clinker cements at less power consumption and at acceptable specific cost of production, including CO2 emissions.
1 Introduction
Cement production is responsible for about 8% of the global CO2 emissions, and the cement industry is presently challenged to reduce process inherent emissions to reach net zero emissions (Tanaka & Stigson 2009, GCCA 2023). Promising pathways for this are technical adaptions in the clinkering process (carbon capture, utilization and storage; alternative and new fuels including hydrogen and biomass), reduction of the clinker factor, and enhanced carbonation to fix CO2 from the atmosphere in concrete structures (Herfort et al. 2010, Matschei 2024). Alternative binder systems with lower CO2 loads are also of interest but may be constrained to special applications by limited availability of raw materials or specials skills needed during application (Sharp et al. 2010). Any pathway to net zero binder production is linked to critical investments for production technology, raw materials sourcing, and the introduction of sustainable cements in existing markets. Threats often overlooked on the products side are unavoidable changes in product properties (compressive strength development, water demand etc.) and significantly increased costs for net zero CO2 cements. The ultimate challenge of the industry will be the production of sustainable cements that fulfill compressive strength and durability requirements at the construction site at acceptable cost.
Reactivity of clinker and cement is of central importance in clinker and cement production. In clinker production, the task is a consistent product with maximum reactivity and minimum CO2 emissions. For cement, the task is maximum consistency of the product to exploit clinker substitution at minimum safety margins. Both tasks are not supported in the current cement plant laboratory setup.
Through the last decades, cement plant quality control successfully integrated instrumental analyzers into their operations (Sandberg et al. 2020). XRF is applied to improve product consistency of the raw meal and XRD verifies the correct clinker mineralogy after the thermal process. Both XRD and XRD, together with laser-based particle sizers, assure correct cement composition and fineness during cement mill operation. Data from quality control improves the consistency of the process. However, the methods in use today in plant control do not assess cement reactivity; so, the numbers only get meaning when linked to data from the physical lab (i.e., compressive strength, water demand, set time etc.).
The results from the physical lab are expensive (materials, labor) and they come far too late to be of use in the daily operation. The manual workload limits compressive strength testing to once a day or less, and compressive strength testing has a significant coefficient of variation due to the manual work, composite sampling, mortar preparation, storage, and testing (ISO 679). Limited data quality and delayed data at low data density exclude the feedback of data from the physical lab to process and product control. The lack of reactivity control close to the process is a major constraint in cement plant process control.
These limitations are today addressed by new software packages, which apply multivariant statistical analyses of large historic data sets combined with fundamental knowledge (i.e., mineralogy, alkali content, TiO2 in GGBFS etc.) to predict compressive strength performance. However, these predictions cannot beat the error of compressive strength testing (ISO 679).
This paper introduces the strengths of automated isothermal calorimeters with examples showing how to quantify cement and clinker hydration kinetics with a focus on the very early hydration reactions (Enders et al 2020). Selected examples highlight the accuracy of automated isothermal calorimetry for various applications, including clinker reactivity and cement reactivity. This new tool can pave the way to new control cycles in cement plants with the aim of producing products with constant reactivity to support the path to net zero CO2. Automated isothermal calorimetry can maintain product consistency in times when plant operation becomes increasingly sensitive to technical, compositional and fuel changes.
1.1 Cement hydration: a process over time
The reactive constituents of cement are clinker (reactivity) and sulfate (set retarder). Clinker is composed of four major crystalline phases: tricalciumsilicate (C3S, alite), dicalciumsilicate (C2S, belite), tricalciumaluminate (C3A) and tetracalciumaluminoferrite (C4AF). Besides these, minor species like free lime (CaOf), periclase (MgO), arcanite (K2SO4) or minor chemical species (i.e., P2O5, alkalis, sulfur, fluorine) further modify the reaction kinetics. These high temperature phases are preserved by quenching the clinker in a clinker cooler to avoid the decomposition that would take place during slow cooling to low temperature conditions. This stored metastable energy is the origin of cement reactivity, and it is released during the hydration of cement.
Once clinker minerals get into contact with water, a series of dissolution and precipitation reactions starts together with a release of heat (Scrivener & Nonat 2011, John & Lothenbach 2023). The dissolution kinetics and precipitation of hydrated phases are controlled by the clinker minerals, their particle size and surface area and the saturation of ionic species in the pore fluid. Hydration reactions of individual clinker phases run on individual time scales but are dependent on each other. The sulfate carrier (gypsum) added plays an important role to avoid formation of calcium-aluminum-hydrates. In a complex pore fluid, hydration and precipitation can be hindered when certain ionic species reach a threshold. For instance, the progress of the C3A reaction is controlled by the sulfate carrier during the first 15 min after water addition. If the chemical balance between C3A dissolution and sulfate carrier is disturbed, the later main hydration of C3S can be delayed (e.g. Pustovcar et al. 2016, Enders et al. 2024, Redondo Soto et al. 2022).
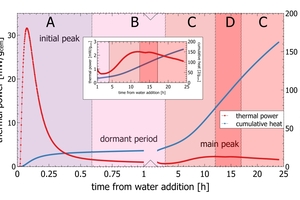
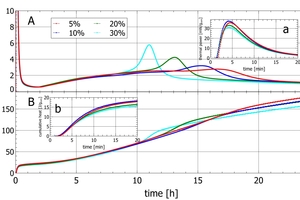
A typical course of grey cement hydration – as seen in isothermal calorimetry – is sketched in Figure 1 (Wadsö et al. 2016). The period after water addition is characterized by a rapid and intense heat release within a few minutes from hydration, mainly due to dissolution of C3A, sulfate and free lime (Figure 1: section A). After 20 – 30 min, the reactions slow down and reach the dormant period, which is a period of quiescence with reduced activity (Figure 1: section B). After a few hours, the acceleration period marks the onset of the main hydration reaction (Figure 1: section C), which is mainly attributed to C3S hydration. The strong increase in the cumulative heat curve is due to the long-lasting rather small heat release. This contrasts with the short but very intense initial peak appearing about 5 minutes after water addition (Figure1: section A). Finally, the hydration fades out during the deceleration period of the main peak (Figure 1: section C). In many cements, the sulfate depletion peak marks a second onset of C3A hydration after the sulfate carrier has been exploited (Figure 1: section D). After the deceleration period, cement hydration continues for long times at lower and lower intensity.
1.2 Analytical tools to monitor the reactivity of cement
Reactivity is a descriptive term combining the driving forces and the kinetics of a chemical reaction. The progress and the intensity of the reaction are controlled by mineral phase composition (the thermodynamic part) and surface area and dissolution rate of individual minerals (the kinetic part), together with additional modifying parameters like minor elements and additives. In the case of cement, hydration reactions are isochemical, except the addition of water needed to dissolve clinker phases prior to formation of hydrated phases.
The analysis of cement reactivity is complicated by the combination of very rapid initial reactions and the long duration of the later main reactions without a well-defined end point (Figure 1). The main methods applied to describe cement hydration are pore solution analyses (batch, continuous), which quantifies the solubility of clinker phases and precipitation of hydrated phases, XRD (batches of stopped samples, in-situ), which qualitatively and quantitatively resolves the formation of hydrated phases and the consumption of clinker phases, and isothermal heat flow calorimetry, which monitors the heat release during the hydration reaction continuously.
Pore solution analyses combine filtration techniques (dead end/dynamic) and high-resolution spectrometry to quantify changes of ionic species in the pore solution as a measure of clinker dissolution and hydrate precipitation over time (Vollpracht et al 2016, Dengler et al 2024). The data can be used to calculate saturation levels for ionic species and to calculate thermodynamic equilibria (Lothenbach et al 2010). With a newly developed cross filtration technique measurements can be made from the first 30 s of hydration until hardening, by a continuous analysis of the pore solution in a bypass system (Dengler et al 2024).
Complementary to pore solution data, in-situ XRD records clinker dissolution and hydrate formation with X-ray diffraction. In-situ XRD collects diffraction data from a single sample with repeated XRD scans during a predefined period. If the duration of individual scans is short, relative to the total time, in-situ XRD can be assumed continuous and only a few minutes during the beginning hydration reaction are missed for paste and sample preparation outside the diffractometer. Within the first hours to a few days the quality of the data is constrained by the low diffraction intensity of weakly crystalline hydrated phases. Often the interpretation of the data is limited to portlandite, ettringite and clinker phase consumption. In-situ XRD data is reported semi-quantitatively; the quantitative interpretation is limited by data quality, possible preferred orientation, available models for the foil (to prevent desiccation), a model for free water (as a sample constituent) and thermal effects during exposure to the high energy X-ray beam. The rather long duration of in-situ measurements blocks the analyzer and causes reduced sample throughput.
Isothermal calorimeters (IC) provide a fully continuous and quantitative record of the heat release of the complete hydration reaction with the most intense reactions occurring withing the first 24 h. Typical applications of IC in the cement field are verifying standard compliance of low heat cements, and analysis and correction of the sulfate balance. As IC does not record the chemistry, the interpretation of the data requires an understanding of hydration reactions or additional data to complete the observations. The IC analyzers typically are available with 8 parallel channels with less constraints on the sample throughput.
In cement production, clinker reactivity – when quantified at all - is assessed in model cements prepared from ground clinker of specified particle size with the addition of sulfate carrier, preparation of mortar prisms, and compressive strength testing. This procedure is complex and time consuming.
Summarizing the information above, no present method gives a complete data set for initial hydration reactions. The constraints arise from the limited amount of solution for pore solution analyses and the low crystallinity of the samples for XRD. Isothermal heat flow calorimetry monitors the reaction continuously but does not describe the hydration reaction in chemical terms.
1.3 How isothermal calorimeters work
When water is added, cement goes through a hydration reaction accompanied by heat release. The rate of this heat release is recorded in an isothermal calorimeter over time. The integration of the signal gives the cumulative heat release as a function of time. Today isothermal calorimeters are widely applied in research to understand effects of cement composition, cement admixtures and cement particle size (Wadsö et al. 2016). In cement plants their usage is typically limited to quantitative assessment of heat release after 7 d to confirm compliance of low heat cements with standards.
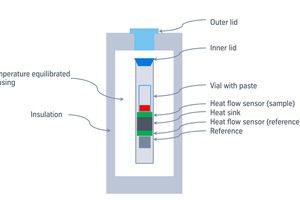
Figure 2 shows schematically the design of an isothermal calorimeter (IC). Usually, eight calorimeters are mounted in a well-insulated and temperature stabilized enclosure. A vial with a paste made from cement and water is placed inside the calorimeter on top of a heat flow sensor with a heat sink below. The heat released during hydration inside the vial is conducted through the heat flow sensor to the heat sink. This heat flow rate is then recorded by the sensor as a proportional voltage. Thermal disturbances are removed by subtracting the signal from a second heat flow sensor with an inert material of approximately similar thermal capacity as the sample.
A critical step of IC is sample preparation and charging of the sample into the calorimeter. When the sample enters the calorimeter, it will not have the same temperature as the calorimeter and any temperature difference – let it be positive (the sample is warmer) or negative (the sample is colder) – will give an initial thermal disturbance, that normally makes it impossible to assess the heat production rate during the early hydration. This thermal disturbance typically lasts for 30-60 min and in practice a user often removes the initial 30-60 min of a measurement.
The problem with the initial thermal disturbance can be overcome by in-situ calorimetry, in which vessels with cement powder are placed and thermostated inside the calorimeter together with water (and admixtures) in syringes. Thermal equilibration of all constituents then occurs inside the calorimeter. After 30-120 min of waiting time, the water is injected on the cement and the paste is mixed inside the calorimeter with a paddle operated from outside the calorimeter. This gives access to the very first hydration reaction, from time zero. Unfortunately, this procedure is time consuming and involves a lot of manual work, the internal mixing is weak and cannot be controlled well, and the quality of mixing cannot be confirmed even after the sample is removed from the calorimeter.
Clinker reactivity has rarely been analyzed by calorimetry due to a required particle size normalization and because clinker without sulfate addition is prone to flash set. Both have critical effects on the signal and the mixing quality.
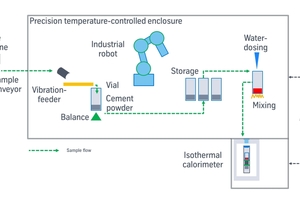
The difficult assessment of the initial hydration reactions initialized a joint development of an automated IC instrument by thyssenkrupp polysius and Calmetrix, that resulted in a product called polabCal (thyssenkrupp polysius). The focus has been minimization of the initial thermal disturbance, a reliable intense mixing process, and a quick start of data collection to catch the initial peak appearing shortly after water addition (Figure 3). Thermal disturbances are minimized by preparing the sample (dosing, weighing, water addition, mixing) within a temperature-controlled enclosure. The key to repeatable measurements is that the temperature (to within a few hundredth of a degree) is the same inside the calorimeter and in the sample preparation enclosure above the calorimeter. The mixing process is outside the calorimeter, to provide intense mixing. Sample handling with warm human hands is replaced by a thermostated gripper of an industrial robot. Quick sample handling and the minimized temperature difference give access to very early hydration reactions. The sample arrives at the heat flow sensor sensor within 40 s after water addition and the duration of the measurement can be chosen by the user (Sugzdaite et al. 2024). This set-up can give reliable calorimetric analyses of early hydration within less than 1 h of sampling of clinker or cement, fast enough to provide signals for process control in cement plants and to enable research of very early hydration of cements.
2 Examples of experiments with an isothermal calorimeter in standard manual mode
We here give examples of calorimetric measurements that assess the kinetics of the main hydration peak. These measurements were conducted with a TAM Air calorimeter (TA Instruments) with a conventional manual sample mixing technique with a spatula (cut off after mixing to avoid material loss) and left in the vial at a w/c ratio of 0.5 (Figure 4). The records of these measurements comprise the dormant period and the main hydration; the initial hydration is not captured.
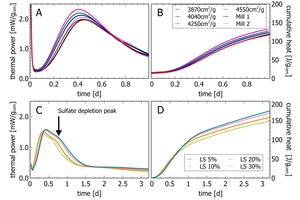
Figure 4 A-B shows the hydration of four cements of different fineness made from the same clinker sulfate carrier mix and ground in a semi-industrial vertical roller mill (VRM) compared to two industrial cements. The target of the study was to align the product quality of cements ground on a future VRM system to the current ball mill. In the calorimetric pattern, the intensity increases with increasing fineness and the peak is shifted to earlier times. By adjusting mill parameters in the semi-industrial mill, it was possible to align the cement performance of both systems as seen by a wide overlap of the mill samples with the semi-industrial grinding at 3870 cm2/g. With increasing fineness, the reaction is accelerated; however, the total energy release (by cumulative heat), which is a fundamental property of the clinker sample, will converge to a single value when the binders are completely hydrated after a very long time.
Figure 4 C-D displays calorimetric analyses of a set of semi-industrial limestone cements with different limestone content at a constant water addition (w/c 0.5). With increasing limestone addition, the reaction is accelerated as seen by the earlier appearance of the main hydration peak. The maximum intensity of the peak decreases due to a lower clinker content. The reasons for these changes are the effect of at least three competing effects: (i) a lower clinker content, (ii) an increased water/clinker ratio and (iii) preferential CSH seed growth on calcite surfaces (e.g. Scherrer & Bellmann 2018, Enders 2010). In the cumulative heat curve, the lower clinker content is seen as an overall lower heat release after the first 24 h of hydration.
In all samples, a small sulfate depletion peak appears at some time after the main peak. Sulfate depletion peaks appear when the SO3 in the pore solution has been depleted. Sulfate depletion can be due to a restarted C3A hydration or the conversion of ettringite (AFt) to monosulfate (AFm). It is not shown in the examples, but excess SO3 shifts the sulfate depletion peak to later times (Pourchet et al 2009). The sulfate depletion peak can be used to optimize sulfate addition to cements (Lerch 1946). Similar experiments are used to improve the understanding of admixture addition (i.e., Cheung 2011).
Isothermal calorimetry supplies important information for product performance as a function of composition and admixture type and content and for instance the compatibility of admixtures added in the cement and in concrete batching. Many researchers also tested isothermal calorimetry for compressive strength prediction (e.g. Boscaro & Flatt 2022, Bentz et al. 2012, Frolich et al. 2014). However, the quality of the correlation is limited by the low repeatability and accuracy of compressive strength testing (ISO 679).
3 Experiments with isothermal calorimeters in automated mode
Automated calorimetry minimizes critical sources of error in calorimetric analyses (human error, thermal disturbance, weak mixing) and makes it possible to study the initial reactions calorimetrically.
The following experiments were conducted on polabCal systems installed in a cement plant and at the thyssenkrupp polysius R&D center. Binder weight was 5 g at a w/c ratio of 0.5. The calorimeters were operated at 23 °C (cement plant) and 20 °C (tk polysius R&D center). Most measurements were recorded for 60-120 min.
Limestone cements in automated isothermal calorimetry:
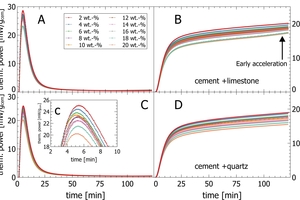
The automated calorimeter in a cement plant was applied to analyze blends prepared from commercial CEM I 42.4 with ground limestone powder (5%, 10%, 20%, 30%). Figure 5 A-B shows the thermal power and the cumulative heat release of these cements during the first 24 h.
The main hydration starts after 2 h after the dormant period as seen by a wide widely overlapping peak for any limestone addition. The main hydration peak is overlaid by a pronounced sulfate depletion peak which appears earlier and becomes smaller with increasing limestone content (Figure 5A). The complex shape of the curves with changing intensity and peak occurrence complicates quantitative analyses.
The initial peak appears in the thermal power graph at the very left side of Figure 5A and cannot be resolved unless the time axis is enlarged (see Figure 5a). The initial peak reaches the maximum after 5 min and fades out into the dormant period after 20 min (cf. Figure 1). The initial heat release is dominated by the C3A content of the clinker fraction in cement. With increasing limestone content, the initial peak becomes smaller due to clinker dilution. However, the position of the maximum thermal power in the initial peak is similar, independent of limestone content. This effect is less pronounced for 5-10% limestone addition, when effects of clinker dilution, changing water/clinker ratio and seeding overlap. (Figure 5A)
The cumulative heat curves (Figure 5B) show the shift of the main peak with shaped curves due to sulfate depletion at 10-18 h after reaction start. The total cumulative heat decreases with increasing limestone content after 24 h due to clinker dilution. The insert (Figure 5B) shows a steady increase of cumulative heat within the first 20 min of hydration. The 5% and 10% limestone results overlap, as clinker dilution and additional free water for clinker hydration compensate each other. Also the short-term cumulative heat of individual samples decreases with increasing limestone content.
The same samples as above were run in 8 replicates each for the initial peak only (Figure 6). The graph shows an excellent repeatability of the initial heat release, for both thermal power and cumulative heat. The peak thermal power for the initial peaks is in all cases significantly higher than for the main peaks (Figure 5, Figure 6). With increasing limestone content, the initial peak intensity (5 min) decreases, except 5-10% where clinker dilution compensates increased water content. The cumulative heat release values decrease with increasing limestone content. In general, the repeatability of the measurements is very good, and the observed statistics are sufficient to use this data also for process control. Main peak intensity data within the first 24 h can be interpreted only, when corrected for sulfate depletion peaks.
3.1 Composite addition
Fineness and cement composition are fixed during cement production, and once fineness is maintained constant it is assumed that the cement performance is also constant. However, clinker reactivity is often unknown, and even if the fineness is kept constant, cement performance might change. In laboratory experiments such reactivity changes can be provoked by clinker substitution with inert materials.
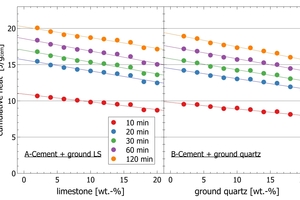
A semi-industrial cement was mixed in steps from 2-20% with a pure limestone powder (Figure 7 A-B) and an industrial quartz powder (Figure 7 C-D). With increasing limestone/quartz content, the thermal power of the initial peak and the cumulative heat within the first 120 min of reaction decrease for both materials.
Figure 8 shows the cumulative heat values for the initial reaction after different integration times, plotted against the limestone content (Figure 8A) and the quartz content (Figure 8B). It is shown that the heat release decreases linearly with decreasing clinker content for each period plotted from 10 to 120 min.
The comparison of individual data points of samples with limestone and quartz powder for thermal power at the peak a cumulative heat surprisingly shows, that even after very short reaction times of 5-10 min only, an additional thermal effect of limestone powder relative to quartz to hydration reactions can be resolved with automated calorimetry (Figures 7-8).
Figure 7B (see arrow) confirms an early onset of the acceleration into the main peak for high limestone addition (18-20 wt.%) by an increasing slope from 100 min of hydration. As this is linked to the limestone content, it is possible that CSH seed formation on calcite surfaces promotes an earlier onset of C3S hydration.
3.2 Sulfate optimization
Sulfate is added to Portland cement to prevent the formation of aluminate hydrates causing flash set. Flash set is avoided by the formation of sulfate-containing compounds like ettringite or monosulfate. In industrial practice, sulfate addition to a cement is verified every couple of weeks or months in a sulfate optimization trial either by compressive strength (repeated test at different sulfate addition) or with isothermal calorimetry by maximizing the main peak for a variation of the SO3 content of the cement (Matschei et al 2019). Both methods are time-consuming and cannot be included in daily quality control. However, in a running cement production, changes in clinker mineral composition or clinker grindability will also instantaneously change C3A surface area and sulfate demand. Such changes require a fast reaction to always use a sufficient sulfate carrier content adapted to C3A surface area, without over-or underdosing the sulfate carrier.
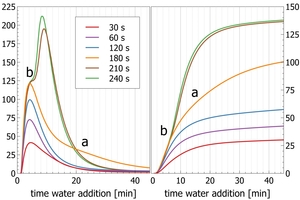
Figure 9 shows the result of an experiment simulating industrial clinker grinding to different fineness. A pre-homogenized clinker sample was interground with 2.9 wt.-% hemihydrate in a polabAPM fine grinding unit (Enders 2003), at high rotational speed (1570 rpm) for 30, 60, 120, 180, 210 and 240 s (Enders et al 2024, Stupperich pers. communication). Increased grinding time increases fineness and surface area, and then also the exposure and rate of dissolution of individual clinker minerals. Most important here is that increased grinding time increases the rate of dissolution of C3A, and increased C3A concentrations need to be matched by additional dissolved SO3 to avoid flash set conditions.
At short grinding times of 30-120 s the initial peak increases as expected with increasing grinding time. From 180 s grinding time, an increased thermal power level appears after 15 min of reaction (point “a” in Figure 9). This feature is also seen in the cumulative heat as the slope does not flatten like seen for samples ground for 30-120 s (point a in Figure 9). It marks the partial or complete consumption of SO3. As a result, C3A is hydrated without sufficient sulfate causing an additional heat release. For samples ground to even higher fineness, sulfate addition is not sufficient to control C3A hydration beyond 4 min of hydration. After 4 min (point “b” in Figure 9), a sigmoidal shape of the curve indicates complete SO3 consumption just before a drastic thermal power increase marks the transfer into flash set conditions. This effect is seen in the cumulative heat curves by a changing slope after 4 min with an increase in cumulative heat, while the low fineness samples show a flat cumulative heat change.
3.3 Clinker reactivity
Isothermal calorimetry analyses of clinker without sulfate carrier addition are difficult due to potential flash set and incomplete mixing. Automated clinker calorimetry offers a fast and powerful external mixing of clinker and water to a homogenous paste. The initial clinker reaction after a few minutes from water addition can then be used to optimize raw meal composition, volatile cycles, and kiln conditions for premium consistent reactivity at minimum emissions and power consumption. The access to the initial peak allows high frequency hourly analyses and if needed corrections to improve clinker consistency.
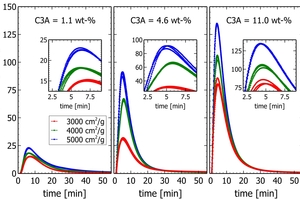
In experiments three clinkers with different C3A content were ground in a lab mill without sulfate addition to 3000, 4000, and 5000 cm2/g (Figure 10). Each clinker sample was then analyzed in triplicates with the automated calorimetry equipment.
Figure 10 shows as expected that the intensity of the initial peak is linked to the C3A content. Then it can be seen, that for each clinker, increasing fineness adds intensity the initial peak. Interestingly this increase seems to be linear with the Blaine value at least for the low and high C3A sample. Finally, as each sample was analyzed three times the exceptional reproducibility of the method is confirmed.
This experiment proves that calorimetric measurements on industrial clinker are possible and that reliable results can be obtained within less than one hour. Depending on the signal used (the initial peak itself or cumulative heat after a certain time), reactivity data is available less than 60 min after sampling at the clinker cooler. In industrial practice, subsequent samples (e.g. hourly) would be compared by reactivity data acquired every hour after normalizing particle size in a fine grinding mill. Fast reactivity data is the key to control clinker reactivity in production.
3.4 Performance enhancers and additives in cement
Many cement plants apply grinding aids or performance enhancers to improve product performance and the economics of the mill operation. Most times, concrete admixtures are also added during the batching process to improve workability and strength development. These substances can interact with the hydration reaction by changing the hydration kinetics. The correct dosing of these agents is important to optimize cement performance without sacrificing the cost targets.
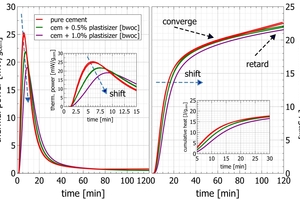
Figure 11 shows in a calorimetric measurement the interaction of a plasticizer with a semi-industrial high fineness CEM I with 4.7 wt.-% sulfate carrier added (50/50 anhydrite/hemihydrate) compared to a blank sample. Except for the high plasticizer addition (1% by the weight of cement), all measurements were recorded in duplicate. For these measurements, the liquid plasticizers were placed in small polyethylene vessels on top of the cement powder inside the vials prior to mixing with water. Just like in concrete batching the individual components cement, plasticizer and water were brought together in the automated mixing process and just before introduction into the calorimeter.
The addition of 1.0% plasticizer delays the initial peak by 3 min and at the same time the intensity of the peak decreases. This confirms the influence of the plasticizer on the C3A reaction, and the overall less intense and wider peak indicates a suppression of the C3A reaction. These observations of the initial peak are also reflected in the cumulative heat with a shift of heat release for a few minutes in the period from 10 to 20 min after water addition. The cumulative heat development converges after one hour during the dormant period, except the high addition which runs in parallel on a lower heat development. After 120 min an increase in the heat release for the blank sample confirms the start of the main reaction while the samples with plasticizer addition are still retarded.
4 Discussion
The polabCal is the first instrument that gives automated accurate and reproducible analyses of the initial peak in isothermal calorimetry (Figures 5-12). Full automation, accurate temperature control together with efficient external mixing are the keys to overcome the constraints of in-situ mixing like the long equilibration times and insufficient control of the mixing process. All this is accomplished with unrivaled accuracy for the initial hydration reactions during the first hour. The high measurement frequency and the rapid data assessment changes isothermal cement calorimetry from mainly descriptive to data driven. In experimental work, speed and automation facilitate the analyses of compete experimental matrices without compromising.
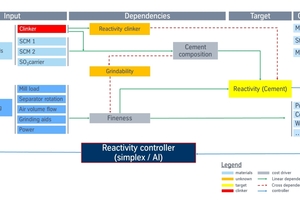
Traditional control cycles in clinker and cement manufacturing with compositional and fineness targets can be replaced by reactivity control on an hourly 24/7 schedule. Reactivity data will be continuously used to optimize the kiln process, the cement composition, and the separator operation. The new control cycles can work like the traditional control cycles with a fixed reactivity setpoint with a limited number of variables (separator, clinker content). In cases with increased complexity of the process (the kiln) or high-volume cement grinding, reactivity data can be linked to compositional and process data in state-of-the-art controls applying artificial intelligence (AI) to unveil hidden factors in large data sets.
The results shown in Figures 5-11 have been made by changing one parameter a time. The results show that small changes give a very early response in the calorimetric data – usually within the first 10 min. This suggests that automated isothermal calorimetry also will be useful in the significantly more complex field of cement plant operation.
Figure 5 shows that the initial peak holds an information equivalent to that of the main peak, but the initial peak itself can be interpreted quantitatively because it is not influenced by the water/clinker ratio. Figure 6 shows that the repeatability of the analyses is good, even for the very early hydration reaction. Figures 7-8 show that the composite material content can be quantified from the calorimetric data of the initial peak – for instance to supervise standard compliance during production. Figure 9 shows that a strong under-sulfatisation is detected within the first 5 min of hydration, and a moderate sulfate deficiency is seen within the first 30 min. This type of analyses will be increasingly important as traditional sulfate carriers like flue-gas desulfurization gypsum are replaced by recycled gypsum or other industrial by-products (Bunzel & Wilczek 2016).
Figure 10 highlights the sensitivity and reproducibility of automated isothermal calorimetry for clinker analyses. Changes in the C3A content and fineness are precisely monitored. Once in a clinker production, variation of clinker reactivity over time can be monitored if the particle size distribution of clinker samples is normalized.
Finally, Figure 11 outlines the impact of plasticizers on the hydration reaction. Those substance are applied in cement plants or in concrete batching and their effect on hydration reactions can be visualized and interpreted much faster and safer with an automated isothermal calorimeter.
In industrial cement production, operators use a fineness target as a set point. The validity of this set point is verified only days or weeks later in compressive strength testing (ISO 679). If the mill would be operated with a reactivity target with the help of a polabCal, the validity of the fineness set point could be challenged every hour. Today two parameters remain unknown during cement grinding; this is clinker reactivity and clinker grindability. If any of those changes, then also the fineness set point in mill operation changes from “correct” to “requires change”. Today’s working practice does not realize changes in clinker reactivity and grindability and fineness targets can only be adjusted when delayed compressive strength data is available.
This calls for a better control of clinker reactivity at the kiln. XRD monitors free lime and phase content of clinker. Clinker reactivity is an unknown. However, it is well known that clinker reactivity is affected by changes of the fuel mix or raw mix chemistry. This identifies an operational benefit from operating the kiln with a reactivity target. Once a deviation from a reactivity target is observed, the kiln operators would take measures to adapt the raw meal or the fuels to return to the target (Sandberg et al. 2020). In practice it is also required to manage the sampling error from granularity of clinker by a reliable sample preparation and a fine grinding procedure (e.g. Enders et al 2024).
Today, reactivity of a cement is controlled by chemical and mineralogy parameters (phase composition, minor elements, grinding aids and performance enhancers) and process parameters (i.e., grindability; grindability of individual minerals). Process control maintains parameters like fineness, cement composition and SO3 constant to reach maximum consistency of the product. In industrial production this constancy is constantly disturbed by varying grindability of the materials, changing process parameters and changing quality of materials (mainly the clinker). Compressive strength results come too late to be useful in the short-term control of the process. Corrections can be applied only after producing considerable volumes already conveyed to the silo. In the field this deficiency is compensated by increased and often costly safety margins to avoid off-spec production.
A reactivity target could be challenged every hour and the required corrections at least for the cement mill are simple – either separator rotation for fineness or clinker content or both can be adjusted to return to the target (Figure 12). A modern mill controller needs to include constraints like the limits from the standard (composition, SO3) and the optimization to specific production costs.
Apart from being used to control cement production, polabCal can be applied in a research environment and used by technical services to rapidly scan large sample matrices for optimized products for specific applications. As an example, a large infrastructure project requires a cementitious binder with defined application properties. Rather than run many mortar and concrete trials with a variation of plasticizer and cement content, this task can be accomplished overnight or over the weekend with multiple polabCal analyses to scan an experimental matrix. Finally, valid solutions are easily identified in a color-coded heat map directing to solutions fulfilling requirements. After the scan, only a limited number of mortar and concrete tests are required to decide on the final recipe.
5 Conclusion
Day by day operators in cement plants experience changes in material and fuel availability and within the next decade new composite materials will add a new degree of complexity to cement manufacturing. This will place new demands on quality control, and for reliable process control and process optimization in increasingly complex plants, the generation of numerical data from the process is crucial. Besides stable chemistry and mineralogy, the reactivity of cements and their sensitivity to clinker quality changes will be increasingly important. New analyzers like the polabCal can then be used to convert reactivity to numbers ready for application in cement plants.
Composites 34 (2012) 11-17 (doi:10.1016/j.cemconcomp.2011.09.004)
213 (https://doi.org/10.1617/s11527-022-02040-5
und Abfälle 3 – Aschen, Schlacken, Stäube und Baurestmassen – ISBN 978-3-944310-28-2
(https://doi.org/10.1016/j. cemconres.2016.06.009).
989 - 996 et al (2009) (doi:10.1016/j.cemconres.2009.07.019)
Second Int. Conference on Concrete Sustainability. Barcelona: Int. Center Numerical Methods Engineering (ISBN 978-84-945077-7-9)
Adv Cem Res 22 (2010): 195-202 (doi: 10.1680/adcr.2010.22.4.195)
Conference in Denver, 28th April 2024 – May 2rd 2024
Cementitious Materials, CRC Press, Boca Raton, 2016, pp. 37–74 (ISBN 13: 978-1-4987-3865-1).