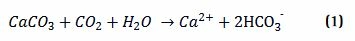Planeteers uses natural processes to decarbonise the industry
Planeteers, a Hamburg-based Climate Tech startup, is pioneering ocean alkalinity enhancement (OAE) for carbon dioxide removal and decarbonisation of hard-to-abate industries, such as lime or cement. The company is aiming to become a global leader in carbon capture and storage by using the natural process of limestone weathering to permanently sequestrate CO2. The approach opens up new markets for limestone, lime and cement companies and creates opportunities for increased circularity, leveraging by-products of mining and processing. So, by transforming natural processes into scalable, science-driven solutions, Planeteers is facilitating the global transition towards net-zero emissions and circular economy while creating new market opportunities.
Who are Planeteers and what do they do
Planeteers envisions a world where natural processes combat climate change by actively removing carbon emissions and restoring our planet’s ecological balance. The company was founded in 2022, and currently has 18 employees. It has a large international partner network of science and industry experts as well as strong links to industry associations such as the German “Bundesverband der Deutschen Kalkindustrie e. V. (BVK)” or the “International Lime Association (ILA)”. The Startup operates from two locations in Hamburg, offering office and a lab space. A first pilot unit with an annual CO2 capture and storage capacity of 60 t is undergoing field testing in Germany, marking significant progress in Planeteers’ mission to combat climate change. The Climate Tech Startup has successfully closed a significant single-digit million seed funding in May, has recently been selected by Airminers, Frontier, Shopify, and Stripe as CDR supplier, and has been rewarded with the prestigious “Hamburger Gründerpreis 2024”.
Scientific process and market opportunities
Climate change is one of the biggest challenges that humanity faces today. In this context, it will be central to how we, as human race, can manage to drastically reduce our CO2 emissions, currently 40 billion t/a. Planeteers has developed a technology to capture CO2, convert it into mineralised water, and store it safely in the ocean – the largest carbon sink on our planet. It builds upon the natural process of limestone weathering: rain picks up CO2 in the air and reacts towards carbonic acid. Once the acid hits limestone on the ground it forms bicarbonate (HCO3-) and calcium (Ca2+). These flow via rivers into the ocean where they remain for 10000+ years. This chemical reaction occurs in nature all the time, everywhere and on a large scale. Unfortunately, time scales of natural limestone weathering are thousands of years. With Planeteers’ technology, the process can be extremely accelerated, so that large amounts of CO2 can be rendered “harmless” within minutes, using the same “ingredients” as in nature.
Here is how it works in practice: CO2 comes to the reactor from exhaust systems (biogenic or fossil sources) of point source emitters and dissolves it in water to carbonic acid (H2CO3), which is then mixed with an alkaline feedstock (e.g. CaCO3) solution. The H2CO3 reacts with limestone slurry, rapidly converting into bicarbonate (HCO3-), following the reaction:
After equilibration with the atmosphere and the local aquatic environment in a diffuser system, it can be released in a controlled way to water bodies which transport the mineralised water to the ocean. There, the CO2 is stored as bicarbonate on a long-term basis (10000-100000 years) helping at the same time to reduce the water acidification locally. Additionally, the system can control the amounts released to ensure that solutions that are discharged to rivers or oceans are in equilibrium with the surrounding waters so that no reversion of the alkalinisation process is triggered, and the CO2 remains stored safely in the form of alkalinity.
Moreover, the impact assessment for the operation of the alkalinity reactor is ensured by a Measurement, Reporting, and Verification (MRV) system, which makes the storage of CO2 and the increase in alkalinity measurable, reportable, and verifiable. The fundamental concept of the MRV system consists of a combination of complex computer modelling and continuous point-by-point comparison with real data through measurements and sampling. Various parameters are constantly measured (e.g. conductivity, pH value, CO2 concentration) in the reactor unit. The alkalinity of the fluid is measured directly before its release. The discharge from the reactor is controlled through the control system and by dilution to ensure that it remains below critical values (established by scientific research) that would reduce the efficiency of our approach. To this end, site-specific, complex software models are used to assess the water flow path and mixing conditions with untreated water downstream. Additionally, selective measurements are conducted to derive maximum values for suitable alkalinity supply from both calculated and measured data points.
By offering a scalable solution for decarbonisation, Planeteers creates a significant market opportunity, particularly in the rapidly growing carbon credits sector. Its approach provides a competitive and diverse tool, projected to be about 2.5 times more cost-efficient in the long run compared to thermal decomposition methods. So one of the company’s main goals in the near future is to sell carbon capture certificates, enabling companies from any sector to offset their emissions and move closer to net-zero targets. This aligns with global climate goals, generating premium carbon credits and offering industries a viable pathway to offset emissions while meeting long-term climate commitments.
Advantages over conventional CCS approaches
Planeteers’ approach offers several economic and environmental advantages over conventional methods like amine scrubbing with storage in depleted gas fields. Unlike energy-intensive amine scrubbing, which requires significant heat to regenerate the chemicals used for capturing CO2, Planeteers’ process relies on natural geochemical reactions between CO2 and limestone. This makes it less energy-demanding, reducing operating costs. Conventional CCS, by contrast, provides no such co-benefit, as CO2 is simply stored underground.
So, Planeteers utilises a point-source focus in its carbon capture approach by directly connecting to emission sources, such as exhaust pipes from industrial facilities, to capture CO2 at the point of generation. The team links their system to industrial point sources, such as factories or power plants, where CO2 emissions are concentrated. Instead of allowing these emissions to escape into the atmosphere, the CO2 is captured directly from the exhaust gases.
This approach is particularly well-suited for decarbonising industries that are geographically close to water sources and produce or utilise limestone-based products, such as the cement, lime, and mining sectors. These industries are traditionally hard to decarbonise due to their high CO2 emissions from both energy use and chemical processes.
Point-Source Capture and Industry Applications
Since Planeteers’ technology captures CO2 at the point of emission and uses limestone as a key input for the accelerated weathering process, industries that already work with limestone or produce limestone by-products can seamlessly integrate this solution. By targeting these industries, Planeteers not only helps to reduce emissions but also creates opportunities for these sectors to actively contribute to both carbon sequestration and ocean health improvement.
Planeteers’ process is highly efficient due to the chemical reactions involved in accelerated weathering, which rapidly convert CO2 into stable bicarbonate forms. These alkaline materials are not only abundant but are also by-products of key industries like cement, lime, and mining. By repurposing these by-products, Planeteers promotes a more sustainable, circular approach by transforming industrial waste into a valuable component for carbon capture.
For industries that produce limestone-based by-products or other alkaline residues, such as slag from steel production or ash from incineration, Planeteers offers a dual benefit: reducing emissions and turning waste into an input for carbon sequestration. This integration of by-products into the carbon capture process lowers operational costs and enhances sustainability by minimising waste, while simultaneously decarbonising industrial operations.
Ambitious plan to unleash the true potential of the ocean
Our long-term vision is to achieve an installed capacity capable of removing 1 Gt/a of CO2 by 2047. Given that annual CO2 emissions are projected to be between 4.2 and 6.2 Gt by 2050, this would allow us to reduce global emissions by approximately one-sixth. And in the shorter term, our goal is to capture 1 million t of CO2 by the end of this decade.
This approach creates a huge market opportunity for lime and cement companies, allowing them to integrate carbon capture into their operations while repurposing their by-products for environmental benefit. Through collaboration with these industries, Planeteers aims to not only combat climate change but also shift the perception of lime and cement producers as key players in transforming their roles from major emitters to environmental leaders.
Planeteers plans to focus on expanding their operations by leveraging the vast potential within the lime and cement industries, which are critical sources of both CO2 emissions and alkaline feedstocks. A key element of this strategy is sourcing feedstock from these industries, including by-products like filter cake, off-spec lime, and kiln dusts. By utilising these materials, Planeteers not only captures CO2 but also helps reduce waste, offering a sustainable solution for these sectors.
Our business model builds upon partnerships, with potential governmental funding support. We offer benefits such as monetising by-products, improving your company’s image, and helping meet regulatory requirements. Join us in advancing decarbonization projects and fighting climate change.