Investigation of hydration mechanisms of alpha and beta hemihydrate
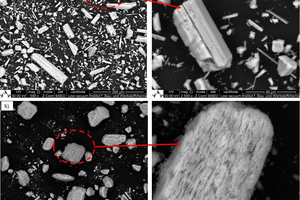
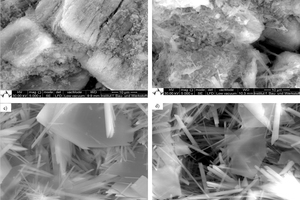
Gypsum-based binders are generally based on the reaction between calcium sulfate hemihydrate (HH) and water to form calcium sulfate dihydrate (DH). Often gypsum can be used as a raw material to obtain hemihydrate. In this study, the obtained alpha hemihydrate consists of large crystals that have more or less lattice defects according to its production process while beta hemihydrate is characterize by a large surface area and fast setting time. The dissolution rate and setting time of α-HH are relatively lower than β-HH, therefore, large dihydrate crystals with fewer branches were formed. The dissolving rate of β-HH and nucleation density of the crated dihydrate was very high due to its high surface area. The gypsum stone which was created from the hydration process out of α-HH is characterized by its higher compressive strength and slower reaction.
1 Introduction
Calcium sulfate hemihydrate [CaCO4 ∙ 0.5H2O(s)] can be found naturally in very dry climate regions in Egypt, Australia, Death valley and Kuwait as bassanite, but in the presence of water or humid atmosphere it can convert to dehydrate [1, 2]. Therefore, the production of hemihydrate industrially has been studied widely through the dehydration process of dihydrate. Based on the dehydration process, the hemihydrate exists in two forms: alpha and beta. Many investigations showed that the symmetry and the water content vary between alpha and beta hemihydrate which might be due to...

![1 a) Represents the solubility curves of gypsum, anhydrite,and hemihydrate while b) represent both nucleation and growth processes [11, 12]](/uploads/images/2020/w300_h200_x532_y233_Materials_Pritzel_Figure_1-236d0eb0081f17a7.jpeg)