Can mixtures of a- and b-hemihydrates be quantified by means of thermoanalysis?
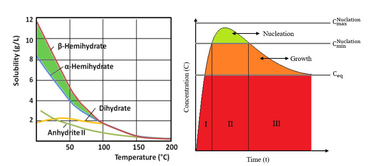
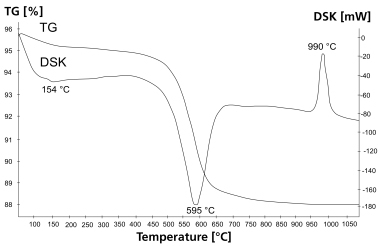
Summary: a-hemihydrate is mainly used for the manufacture of high-quality products of gypsum building material. The b-type is used for the production of articles made in bulk. The question as to what extent mixtures of a- and b-hemihydrate will already result from manufacturing processes is still open, because there is no suitable detection technique. Up to now thermal analysis has been taken into consideration for the quantification, since a- and b-CaSO4 ∙ 0.5 H2O differ due to an exothermic effect at different temperatures. Depending on the crystal shape and size, however, this effect cannot be measured uniformly. The reason is a kinetics of the phase transition with increasing temperature that is depending on the crystallite form. Therefore, thermal analysis is excluded for the quantitative analysis of a-/b-mixtures.
1 Introduction
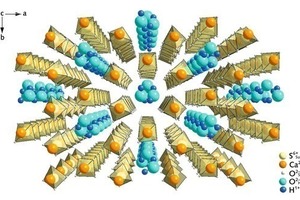
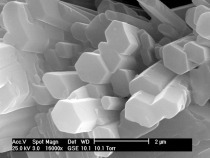
Calcium sulphate-hemihydrate (CaSO4 ∙ 0.5 H2O) is manufactured from gypsum (CaSO4 ∙ 2 H2O) by partial dewatering in two different ways. In the presence of high partial water vapour pressures at temperatures between 80 °C and 120 °C (hydrothermally in a steam autoclave), but also in acids or salt solutions at temperatures above 45 °C, the hemihydrate is formed as well-developed hexagonal prisms (Fig. 1a). At low partial water vapour pressures, e. g. during the dewatering of gypsum in air or in a vacuum at temperatures between 45 °C and 200 °C, the so-called beta-form of the...