Effect of chemical activator on early hydration of the fly ash-cement system under steam curing
In this paper, the effects of calcium formate (CF) and sodium sulfate (SS) on the early compressive strength, hydration characteristic and microstructure of the fly ash-cement system were investigated and compared by the tests of mechanical properties, XRD, DSC-TG, MIP, SEM and FTIR. Results show that both the addition of CF and SS can significantly increase the early strength of the fly ash-cement system, and the optimal mixing amounts of CF and SS are 1.5% and 2.0%, respectively; the incorporation of CF and SS can increase the hydration rate of the fly ash-cement system, promote the generation of hydration products, refine the pore structure, reduce the porosity and total pore volume, and transform harmful and very harmful pores into less harmful and harmless pores. Moreover, the polymerization degree of Si-O tetrahedron can be increased by adding CF, while the addition of SS could only increase the C-S-H gels generation, but does not change the polymerization degree and chain length of C-S-H. Such results can provide reference for the application of chemical activators in high volume fly ash-cement composites.
1 Introduction
Reducing carbon emissions in the building sector is an indispensable part of achieving the goal of “carbon peak and carbon neutrality” [1]. In the process of cement production and use, huge carbon emissions would be generated [2]. The incorporation of industrial solid waste, such as fly ash, into concrete can not only reduce the use of cement and environmental pollution, but also improve the later strength and durability of concrete [3-5]. However, the incorporation of a large amount of fly ash is not conducive to the development of early mechanical properties of concrete [6, 7]. Steam curing is a common method of maintenance in the production of precast concrete components, which can promote cement hydration, improve the demolding strength, and thus speed up the mold turnover [8, 9]. However, when the fly ash content is large, steam curing alone cannot meet the needs of concrete early strength in practical engineering.
The use of chemical activator is also another effective way to increase the strength of cement composites [10, 11]. Calcium formate (CF) is a kind of organic early strength agent, which is weakly acidic after ionization in water, can reduce the pH value of the liquid phase, increase the hydration reaction rate of tricalcium silicate, and accelerate the coagulation and hardening of paste. More-over, CF can provide Ca2+ to the paste, accelerate the precipitation of hydrated calcium silicate and calcium hydroxide, improve the solid ratio in the paste, and facilitate the construction of the cement stone structure, thus improving the strength of the specimen [12-14]. Sodium sulfate (SS) is another widely used early strength agent for cement-based materials, which has the dual excitation effects of alkali and sulfate [15-17]. For one thing, the incorporation of Na2SO4 improves the alkalinity of the system, which can effectively promote the depolymerization and disintegration of the vitreous network of fly ash, releases the soluble active silica and alumina inside, and makes them react with the calcium hydroxide in the hydration products of cement, thus speeding up the hydration reaction rate of fly ash cement. For another thing, SS reacts with Ca(OH)2 generated by cement hydration to produce CaSO4 · 2H2O, which is highly dispersed in cement-based materials. The reaction rate of CaSO4 · 2H2O with C3A is much faster than that of gypsum mixed in cement production with C3A, which can quickly generate aciculate calcium sulfoaluminate hydrate and form an early skeleton. Simultaneously, the decrease of Ca(OH)2 concentration in cement paste is conducive to the C3S hydration reaction.
At present, there have been a lot of studies on the influence of calcium formate and sodium sulfate on the early strength of the fly ash cement system, but there are few studies on the comparison of the two chemical activators, especially under the condition of steam curing. Based on this, the effects of calcium formate and sodium sulfate on the early compressive strength of the fly ash-cement system and their mechanisms were investigated and compared in this paper by the tests of mechanical properties, XRD, DSC-TG, MIP, SEM and FTIR, in order to provide reference for the application of chemical activators in high volume fly ash-cement composites.
2 Experiment
2.1 Raw materials
P·O 42.5 cement and grade II fly ash (FA) were used in all mixes, and their chemical compositions are shown in Table 1. Calcium formate (CF) with a purity of 98% and sodium sulfate (SS) of the analytical grade were used as chemical activator. Wuhan tap water was used as the mixing water.
2.2 Sample preparation
The mix proportions of the pastes are shown in Table 2. The water-binder ratio of the sample is 0.38, the content of fly ash is 30% of the mass of the total binders, and the mass fraction of calcium formate and sodium sulfate were related to the amount of binders, which are 0.5% ~ 2% and 1% ~ 2.5% of the total mass of cement and FA. The prismatic molds of 40 mm × 40 mm × 160 mm were used to prepare the paste samples and the compressive strength test was performed after the pastes were cured by steam at 60 °C for 12 h.
In order to prepare the samples for the microscopic tests, the small fragments obtained from the central part of the pastes were soaked in anhydrous ethanol for 3 d to terminate hydration, and then dried at 60 °C in a vacuum drying oven. After that, some were ground into powder for XRD, DSC-TG and FTIR tests, and the others were taken for SEM and MIP tests.
2.3 Test procedure
A YAW-200/300 automatic pressure testing machine was used to test the compressive strength of the samples according to GB/T 17671-2021 [18]. The phase composition and crystal structure of the samples were measured by the STA449C Advance X-ray diffraction. The differential thermal analysis was performed by STA449C/3/G synchronous thermal analyzer. The porosity and pore size distribution of the samples were measured by PoreMaster-33 mercury injection porosimeter. The surface morphology and structure of the samples were observed by JSM-6610 scanning electron microscopy. The chemical structure of the samples was analyzed by Nicolet iS50 Fourier transform infrared spectroscopy.
3 Results and discussion
3.1 Compressive strength
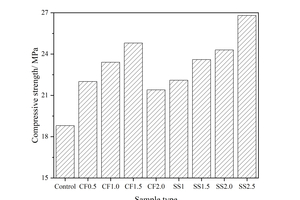
Figure 1 shows the effect of different chemical activators on the strength of the fly ash-cement system under steam curing. It can be seen from Fig. 1 that under steam curing conditions, the incorporation of CF can significantly improve the early compressive strength of the fly ash-cement system, and with the increase of CF content, the strength of the sample first increases and then decreases. When the CF content is 1.5%, the strength reached the maximum. When the CF content is 2.0%, the compressive strength decreases slightly, but is still higher than that of the control sample. This is because CF can accelerate the hydration of the fly ash-cement system, promote the formation of hydration products, increase the solid ratio in the paste, and thus improve the early strength of the sample [19, 20]. However, the addition of too much CF would consume a lot of water and make the paste become viscous, which is not conducive to the structural construction [21]. Therefore, the early compressive strength of the paste does not increase further when the CF content is large.
It is also obvious from Figure 1 that the addition of SS can significantly increase the early strength of the sample, and within the dosage range of 1.0%~2.5%, the higher the SS content, the greater the increase of strength. According to the GB175-2020 standard, the SO3 content in cement should be less than 3.5%. Based on the SO3 content in cement and FA in Table 1, it can be calculated that the dosage of 2.0% SS makes SO3 account for 3.4% of the binders, and 2.5% SS makes SO3 account for 3.7% of the binders. As a consequence, the optimum content of SS is 2% in this study.
3.2 XRD analysis
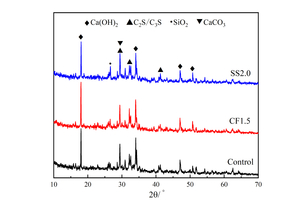
Figure 2 shows the XRD spectra of Control, CF1.5 and SS2.0 samples at 12 h under steam curing.
It can be seen from Figure 2 that, before and after the addition of chemical activators, there is no significant change in the position of diffraction peaks of the XRD pattern of the sample at 12 h, that is, the addition of chemical activators does not affect the types of hydration products, and the main phases are calcium hydroxide (CH), calcium silicate (CS: C3S/C2S), quartz (SiO2) and calcium carbonate (CaCO3).
As shown in Figure 2, the diffraction peak intensity of CH in CF1.5 is significantly higher than that in the Control sample, while the peak intensity of CS in CF1.5 is somewhat lower, indicating that CH content in hydrated products is increased and the unhydrated cement clinker is reduced. The reasons for this phenomenon are as follows [22, 23]: First, the diffusion rate of HCOO- in CF is faster than Ca2+, which can quickly pass through the initial hydration layer on the surface of C2S and C3S, reduce the nearby pH value, improve the hydration reaction rate, and promote the generation of hydration products; Second, the incorporation of CF increases the concentration of Ca2+ in the liquid phase, reduces the nucleation barrier of CH crystals, and increases the precipitation rate of CH. In addition, although CH is consumed by pozzolanic reaction of FA, the CH consumption is lower than the CH production by the hydration of cement, so the intensity of the CH diffraction peak is still higher than that of the Control sample.
In addition, the diffraction peak intensities of CH and CS in the SS2.0 sample show a similar trend to those in the CF1.5 sample, mainly because SS is a strong electrolyte, SO42- and Na+ can react with CH in the paste to form CaSO4 · 2H2O and NaOH, which increases the pH value and the C3A solubility. Simultaneously, the ion concentration difference inside and outside the C3S coating layer increases, which accelerates the early hydration of C3S [24].
3.3 DSC-TG analysis
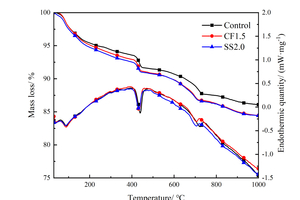
In order to quantitatively analyze the mass changes of each phase of the hydration products, the samples of Control, CF1.5 and SS2.0 were analyzed by a thermal analyzer, and the DSC-TG curves as shown in Figure 3 were obtained.
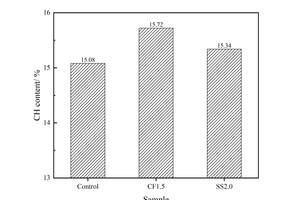
As shown in Figure 3, there are three obvious endothermic peaks on the DSC curves, corresponding to three obvious mass changes in TG. Specifically, the mass change corresponding to the endothermic peaks at about 105 °C results from the evaporation of free water and decomposition of C-S-H and AFt; The mass change corresponding to the endothermic peak around 400~500 °C is related to the CH decomposition. The mass change corresponding to the endothermic peak around 700 °C is caused by the CaCO3 decomposition. Considering that CaCO3 is the carbonization product of CH, the amount of CH (%) can be determined by Formula (1) [25], and the calculation results are listed in Figure 4.
CH% = m(CH) × 4.11 + m(CC) × 1.68⇥(1)
Where m(CH) and m(CC) represent the mass loss of CH and CaCO3 decomposition respectively, and 4.11 and 1.68 represent the relative molecular mass ratio of CH to H2O and CH to CO2 respectively.
As shown in Figure 4, whether CF or SS is added, the amount of CH in the sample would be increased, which is consistent with the XRD results. However, the amount of CH produced in the sample mixed with SS is slightly lower than that in the sample mixed with CF, which may be because the pH of the liquid phase is increased after the addition of SS, the acid oxide is released by the dissolution of the vitreous body of the fly ash, and the activity of the fly ash is stimulated and some CH is consumed in the pozzolanic reaction. While CF dissolved in water is weakly acidic, and the pH in the paste is lower than that of the sample mixed with SS, so the excitation effect of fly ash in CF1.5 is worse than that in SS2.0, resulting in the CH content of SS2.0 sample being lower than that of CF1.5 sample.
3.4 MIP analysis
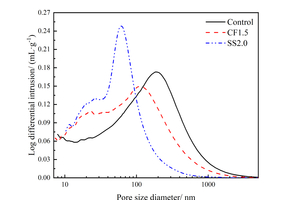
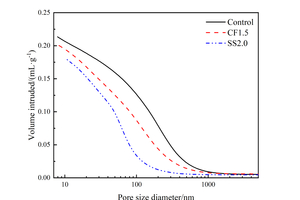
Figure 5 shows the pore size distribution results of the sample with chemical activators at 12 h under steam curing, and the specific pore structure parameters are listed in Table 3.
As Figure 5(a) shows, compared with the Control sample, the most probable apertures of both CF1.5 and SS2.0 are smaller, indicating that the pore size can be refined by mixing CF or SS, and the effect of SS is superior to CF. As it can be seen from Figure 5(b) that, the order of total pore volume of the samples is: Control>CF1.5>SS2.0, which is because chemical activators added in the fly ash-cement system improve the hydration degree of the paste, and the hydration products fill the pores, resulting in a decrease in total pore volume compared with the control group. The most probable aperture of the SS2.0 sample is lower than that of the CF1.5 sample because SS has a better excitation effect on fly ash activity than CF, and more hydration products are generated in the paste to fill the pores.
Moreover, it is obvious from Table 3 that, the amount of very harmful pores (>200nm) and harmful pores (50~200nm) in CF1.5 and SS2.0 samples is lower compared with that of the Control sample, while the amount of less harmful pores (20~50nm) and harmless pores (<20nm) in CF1.5 and SS2.0 samples is higher, indicating that the incorporation of CF and SS can transform very harmful pores and harmful pores into less harmful pores and harmless pores, which is conducive to the refinement of the paste pore size. The optimization of pore structure can explain the change of compressive strength, and the reduction of porosity and total pore volume and the refinement of pore structure can improve the compressive strength [26, 27].
3.5 SEM analysis
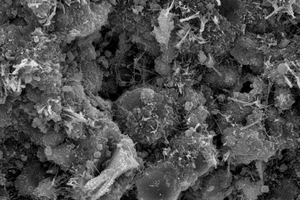
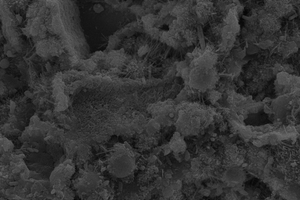
Figure 6 shows the SEM images of fly ash-cement paste mixed with chemical activator under steam curing at 12 h. From Figure 6(a), it is obvious that the fly ash microspheres in the control sample are no longer smooth, there are some hydration products on the surface of the particles, and AFt in the paste is mostly needle rod shaped, which is bonded with other hydration products on the fly ash balls and filled between the pores of the particles, forming a relatively complete network structure, but the degree of hydration is low, the structural pores are large, and the overall structure is loose. It can be seen in Figure 6(b) that, after the incorporation of CF, the hydration degree of fly ash increases, C-S-H gels are formed on the fly ash microspheres, and AFt and C-S-H gels fill the pores between fly ash, and the hydration products increase compared with the control sample, the distribution is more disorderly, the porosity of the paste decreases, and the overall structural compactness is improved. As shown in Figure 6(c), after SS is incorporated into the fly-ash cement system, AFt in the paste is cross-constructed, more C-S-H gels generate and cover on the surface of the fly ash microsphere, some pores are filled with fine gypsum, and thus the structure is more compact. These may be due to the fact that SO42- reacts with CH to form calcium sulfate and gypsum, and simultaneously SO42- can displace part of SiO42-, which can react with Ca2+ again in the liquid phase to form C-S-H gels [28], and thus improve the hydration degree of the pastes.
3.6 FTIR analysis
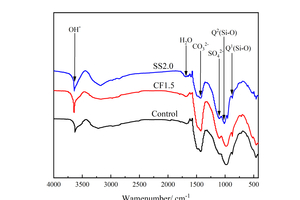
The FTIR spectra of Control, CF1.5 and SS2.0 samples under steam curing for 12 h are shown in Figure 7.
As shown in Figure 7, the S-O vibration peak appears at around 1100 cm-1, which is due to the presence of AFt in hydration products. Q1 and Q2 vibration peaks related to C-S-H appear in all samples, and the Q2 vibration peak of CF1.5 (988 cm-1) migrates to a higher wave number than the Control sample (970 cm-1), showing that the polymerization degree of Si-O tetrahedron can be increased by adding CF. The Q2 vibration peak of SS2.0 sample is similar to that of the control sample, which may be because the addition of SS could increase the C-S-H gels generation of the fly ash-cement system, but does not change the polymerization degree and chain length of C-S-H. The absorption peak near 1453 cm-1 is the vibration peak of CO32-, and the OH- vibration peak related to CH is located at 3643 cm-1. Compared with the control sample, the OH- vibration peaks increase after the addition of chemical activators, indicating that the hydration degree of the fly ash-cement system can be improved after the addition of chemical activators.
4 Conclusion
The main conclusions of this study are shown as follows:
1. Both the addition of CF and SS can significantly increase the compressive strength of the pastes under steam curing at 12 h, and the optimum dosages of CF and SS are 1.5% and 2.0%, respectively.
2. Whether CF or SS is added, it would increase the amount of CH in hydration products, and decrease the amount of calcium silicate in cement clinker. In addition, CF has a more significant effect on CH production than SS.
3. Under steam curing condition, the addition of CF and SS can increase the hydration rate of the fly ash-cement system, promote the generation of hydration products, refine the pore structure, reduce the porosity and total pore volume, and transform harmful and very harmful pores into less harmful and harmless pores.
4. The addition of CF increase the polymerization degree of Si-O tetrahedron, while the addition of SS could increase the C-S-H gels generation of the fly ash-cement system, but does not change the polymerization degree and chain length of C-S-H.
5 Acknowledgement
Financial supports from the Innovation and Entrepreneurship Training Program of Hubei University Students (S202210495067) and Science and Technology Research Program of Hubei Province Education Department (Q20191706) are gratefully acknowledged.