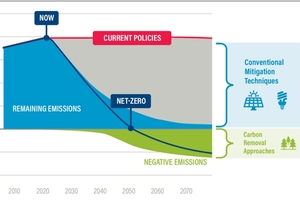
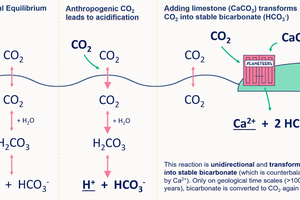
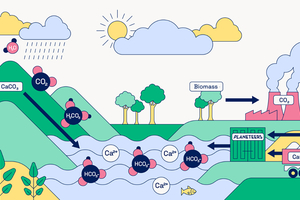
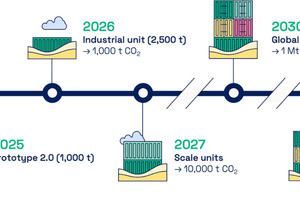
REFERENCES
[1] Adams, E.E., Caldeira, K., 2008. Ocean Storage of CO2. Elements 4, 319–324. https://doi.org/10.2113/gselements.4.5.319
[2] Amann, T., Weiss, A., Hartmann, J., 2014. Inorganic Carbon Fluxes in the Inner Elbe Estuary, Germany. Estuaries and Coasts 1–19. https://doi.org/10.1007/s12237-014-9785-6
[3] Bach, L.T., Gill, S.J., Rickaby, R.E.M., Gore, S., Renforth, P., 2019. CO2 Removal With Enhanced Weathering and Ocean Alkalinity Enhancement: Potential Risks and Co-benefits for Marine Pelagic Ecosystems. Frontiers in Climate 1. https://doi.org/10.3389/fclim.2019.00007
[4] Battaglia, G., Steinacher, M., Joos, F., 2016. A probabilistic assessment of calcium carbonate export and dissolution in the modern ocean. Biogeosciences 13, 2823–2848. https://doi.org/10.5194/bg-13-2823-2016
[5] Berner, R. A. (1975). The role of magnesium in the crystal growth of calcite and aragonite from sea water. Geochimica et Cosmochimica Acta, 39(4), 489-504.
[6] Buesseler, K., Leinen, M., Ramakrishna, K., 2022. Removing carbon dioxide: first, do no harm. Nature 606, 864–864. https://doi.org/10.1038/d41586-022-01774-0
[7] Caserini, S., Pagano, D., Campo, F., Abbà, A., De Marco, S., Righi, D., Renforth, P., Grosso, M., 2021. Potential of Maritime Transport for Ocean Liming and Atmospheric CO2 Removal. Frontiers in Climate 3. https://doi.org/10.3389/fclim.2021.575900
[8] Chen, Z., Auler, A.S., Bakalowicz, M., Drew, D., Griger, F., Hartmann, J., Jiang, G., Moosdorf, N., Richts, A., Stevanovic, Z., Veni, G., Goldscheider, N., 2017. The World Karst Aquifer Mapping project: concept, mapping procedure and map of Europe. Hydrogeol J 25, 771–785. https://doi.org/10.1007/s10040-016-1519-3
[9] Emerson, S., Hedges, J., 2008. Chemical Oceanography and the Marine Carbon Cycle, 1st ed. Cambridge University Press. https://doi.org/10.1017/CBO9780511793202
[10] Friedlingstein, P., O’Sullivan, M., Jones, M.W., Andrew, R.M., Gregor, L., Hauck, J., Le Quéré, C., Luijkx, I.T., Olsen, A., Peters, G.P., Peters, W., Pongratz, J., Schwingshackl, C., Sitch, S., Canadell, J.G., Ciais, P., Jackson, R.B., Alin, S.R., Alkama, R., Arneth, A., Arora, V.K., Bates, N.R., Becker, M., Bellouin, N., Bittig, H.C., Bopp, L., Chevallier, F., Chini, L.P., Cronin, M., Evans, W., Falk, S., Feely, R.A., Gasser, T., Gehlen, M., Gkritzalis, T., Gloege, L., Grassi, G., Gruber, N., Gürses, Ö., Harris, I., Hefner, M., Houghton, R.A., Hurtt, G.C., Iida, Y., Ilyina, T., Jain, A.K., Jersild, A., Kadono, K., Kato, E., Kennedy, D., Klein Goldewijk, K., Knauer, J., Korsbakken, J.I., Landschützer, P., Lefèvre, N., Lindsay, K., Liu, J., Liu, Z., Marland, G., Mayot, N., McGrath, M.J., Metzl, N., Monacci, N.M., Munro, D.R., Nakaoka, S.-I., Niwa, Y., O’Brien, K., Ono, T., Palmer, P.I., Pan, N., Pierrot, D., Pocock, K., Poulter, B., Resplandy, L., Robertson, E., Rödenbeck, C., Rodriguez, C., Rosan, T.M., Schwinger, J., Séférian, R., Shutler, J.D., Skjelvan, I., Steinhoff, T., Sun, Q., Sutton, A.J., Sweeney, C., Takao, S., Tanhua, T., Tans, P.P., Tian, X., Tian, H., Tilbrook, B., Tsujino, H., Tubiello, F., Van Der Werf, G.R., Walker, A.P., Wanninkhof, R., Whitehead, C., Willstrand Wranne, A., Wright, R., Yuan, W., Yue, C., Yue, X., Zaehle, S., Zeng, J., Zheng, B., 2022. Global Carbon Budget 2022. Earth Syst. Sci. Data 14, 4811–4900. https://doi.org/10.5194/essd-14-4811-2022
[11] Gately, J.A., Kim, S.M., Jin, B., Brzezinski, M.A., Iglesias-Rodriguez, M.D., 2023. Coccolithophores and diatoms resilient to ocean alkalinity enhancement: A glimpse of hope? Sci. Adv. 9, eadg6066. https://doi.org/10.1126/sciadv.adg6066
[12] Gattuso, J.-P., Frankignoulle, M., Bourge, I., Romaine, S., Buddemeier, R.W., 1998. Effect of calcium carbonate saturation of seawater on coral calcification. Global and Planetary Change 18, 37–46. https://doi.org/10.1016/S0921-8181(98)00035-6
[13] Gim, B.-M., Hong, S., Lee, J.-S., Kim, N.-H., Kwon, E.-M., Gil, J.-W., Lim, H.-H., Jeon, E.-C., Khim, J.S., 2018. Potential ecotoxicological effects of elevated bicarbonate ion concentrations on marine organisms. Environmental Pollution 241, 194–199. https://doi.org/10.1016/j.envpol.2018.05.057
[14] Gruber, N., Clement, D., Carter, B.R., Feely, R.A., Van Heuven, S., Hoppema, M., Ishii, M., Key, R.M., Kozyr, A., Lauvset, S.K., Lo Monaco, C., Mathis, J.T., Murata, A., Olsen, A., Perez, F.F., Sabine, C.L., Tanhua, T., Wanninkhof, R., 2019. The oceanic sink for anthropogenic CO2 from 1994 to 2007. Science 363, 1193–1199. https://doi.org/10.1126/science.aau5153
[15] Hartmann, J., Moosdorf, N., 2012. The new global lithological map database GLiM: A representation of rock properties at the Earth surface. Geochem Geophy Geosy 13. https://doi.org/10.1029/2012gc004370
[16] Hartmann, J., Suitner, N., Lim, C., Schneider, J., Marín-Samper, L., Arístegui, J., Renforth, P., Taucher, J., Riebesell, U., 2023. Stability of alkalinity in ocean alkalinity enhancement (OAE) approaches – consequences for durability of CO2 storage. Biogeosciences 20, 781–802. https://doi.org/10.5194/bg-20-781-2023
[17] Henderson, P.G., Rickaby, R., Bouman, Heather, 2008. Decreasing atmosphere CO2 by increasing ocean alkalinity. University of Oxford, Department of Earth Sciences and The James Martin 21st Century Ocean Institute.
[18] Ho, D.T., Bopp, L., Palter, J.B., Long, M.C., Boyd, P., Neukermans, G., Bach, L., 2023. Chapter 6: Monitoring, Reporting, and Verification for Ocean Alkalinity Enhancement. State of the Planet Discussions 2023, 1–15. https://doi.org/10.5194/sp-2023-2
[19] Ilyina, T., Wolf-Gladrow, D., Munhoven, G., Heinze, C., 2013. Assessing the potential of calcium-based artificial ocean alkalinization to mitigate rising atmospheric CO2 and ocean acidification. Geophysical Research Letters 40, 2013GL057981. https://doi.org/10.1002/2013GL057981
[20] IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2391 pp. doi:10.1017/9781009157896.
[21] Lackner, K.S., 2003. A Guide to CO2 Sequestration. Science 300, 1677–1678. https://doi.org/10.1126/science.1079033
[22] Mackenzie, F.T., Garrels, R.M., 1966. Chemical mass balance between rivers and oceans. American Journal of Science 264, 507–525. https://doi.org/10.2475/ajs.264.7.507
[23] Morse, J. W., & He, S. (1993). Influences of T, S and pCO2 on the pseudo-homogeneous precipitation of CaCO3 from seawater: implications for whiting formation. Marine Chemistry, 41(4), 291-297.
[24] Oschlies, A., Bach, L., Rickaby, R., Satterfield, T., Webb, R.M., Gattuso, J.-P., 2023. Climate targets, carbon dioxide removal and the potential role of Ocean Alkalinity Enhancement. State of the Planet Discussions 2023, 1–11. https://doi.org/10.5194/sp-2023-13
[25] Pytkowic, R. (1973) Calcium-Carbonate Retention in Supersaturated Seawater. American Journal of Science, 273, 515-522.
[26] Renforth, P., Henderson, G., 2017. Assessing ocean alkalinity for carbon sequestration. Reviews of Geophysics. https://doi.org/10.1002/2016rg000533
[27] Schulz, K.G., Bach, L.T., Dickson, A.G., 2023. Seawater carbonate system considerations for ocean alkalinity enhancement research. State of the Planet Discussions 2023, 1–24. https://doi.org/10.5194/sp-2023-12
[28] Sharifian, R., Wagterveld, R.M., Digdaya, I.A., Xiang, C., Vermaas, D.A., 2021. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781–814. https://doi.org/10.1039/D0EE03382K
[29] Yang, B., Leonard, J., & Langdon, C. (2023). Seawater alkalinity enhancement with magnesium hydroxide and its implication for carbon dioxide removal. Marine chemistry, 104251.